Analysis of Cerebrospinal Fluid Dynamics - I. WWW
Cerebrospinal fluid (CSF) dynamics in the human central nerve system (CNS) is an extremely interesting topic in bio-fluid mechanics. The disorders of the CNS components disrupt the CSF pulsatility which is involved in a number of vital functions. The CFD analysis can be used to quantify the functional interactions of the CNS member. The numerical simulations of CSF filled in brain ventricles and subarachnoid spaces (SAS) are performed to build up a basis for testing and optimizing the CNS therapeutics via understanding of the CSF pathophysiology. In this post a recent collaboration of the SimLab - Highly Scalable Fluids and Solids Engineering at the Jülich Supercomputing Centre with Korea University Medical Center is presented.
What is the CSF?
The CSF surrounds a brain and a spinal cord such that the CSF fills the cerebral ventricles as well as the cranial and SAS. Not only the mechanical protection for the central nerve system, the CSF transports nutrients, neuroendocrine substances, and the removal of toxic metabolites to preserve the chemical environment of the brain (Linninger et al. 2016). The fluid dynamic properties, e.g., density and viscosity, are close to water. Several prominent disease states represent disorder of the CSF dynamics which includes an increased intracranial pressure (ICP) by hydrocephalus (Balédent et al. 2004) and decreases in the flow and pulsatility by Chiari malformations (Armonda et al. 1994). Under the abnormal CSF conditions, although various symptoms can occur, the specific etiologies are hardly understood to identify the biomechanical interactions between the components of the CNS.
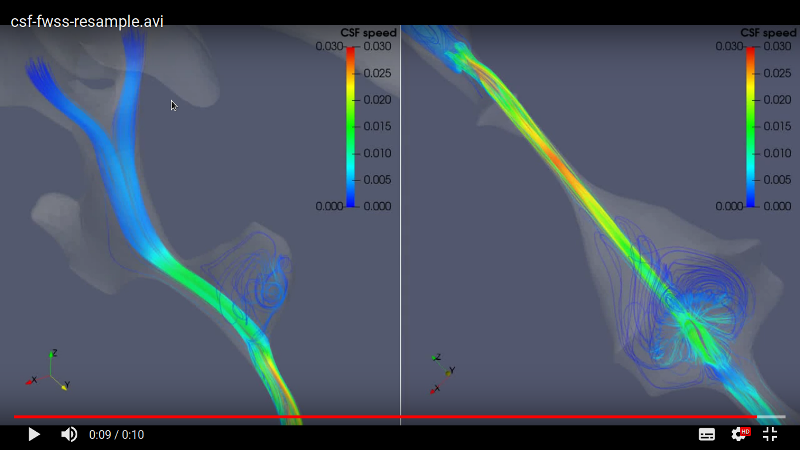
The CSF flow pulsates through two lateral ventricles which are connected with the third ventricle. A thin channel, i.e., the aqueduct of Sylvius, communicates with the third and the fourth ventricle. The CSF from the fourth ventricle is transferred to the cranial and spinal SAS. The clinical decision-making is difficult when the symptom occurs with no disease characteristics, e.g., normal pressure hydrocephalus. The recent studies have been oriented to develop automated algorithms detecting the changes in the ventricle system (Ambarki et al. 2012, Saele et al. 2015).
In clinical applications the CSF circulation models consider the pressure dynamics and the dynamics of CSF flow and cerebral blood flow. The quantitative assessment provides robust evidences in judging a patient care when the clinical parameters are subtle and ambiguous (Czosnyka et al. 2011, Linninger et al. 2016). The mechanical CSF model is based on the fundamental conservation law of mass, momentum, and chemical reactions (Gupta et al. 2010, Tangen et al. 2015, Khani et al. 2018). The approaches using CFD can simulate realistic CSF flows to predict the patient-specific scenarios. The present study aims to establish a basis to test and optimize a clinical therapy via the anatomically accurate simulation based on a robust numerical procedure.
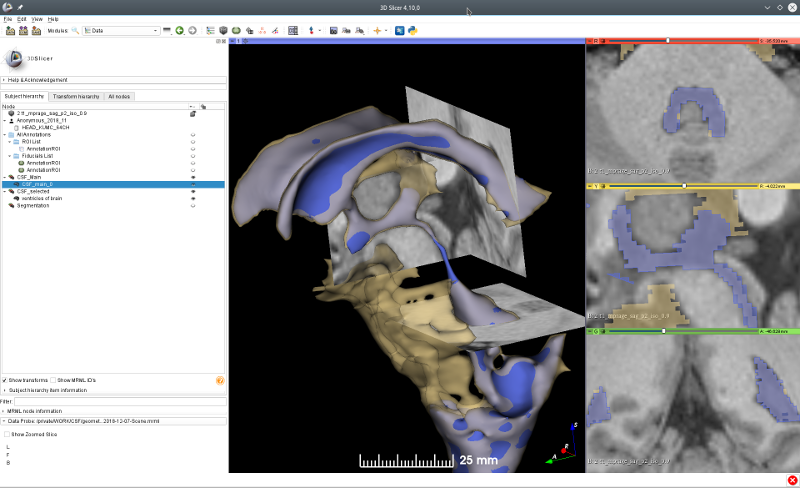
The three-dimensional images obtained by magnetic resonance imaging (MRI) measurement provide the structures of ventricles and the SAS. For a normal healthy human the CSF speed ranges in several centimeters per second. The typical Reynolds number is in the order of $\mathcal{O}(10^2)$. However, the physiological mechanisms of the CSF circulation develop vortical flow structures which exhibit a turbulent-like motion induced by the difference in systolic and diastolic velocities and the interactions of micro-structures with fluids (Tangen et al. 2015, Khani et al. 2018). Furthermore, to resolve the micro-structures, like nerve roots and trabeculae, a short spinal segment already demands a significant increase of the computational cost (Tangen et al. 2015).
Why do I need HPC to understand CSF dynamics?
At a first glance it seems strange to apply a high-performance computing technique to a low Reynolds number flow, i.e., the flow in the human brain ventricles moves at very slow speeds less than dozens of centimeters per second. However, to achieve a therapeutic basis, the complex anatomical geometries as well as the CSF pulsatility require spatially and temporarily high-resolution computations based on the high-performance computing (HPC) technique. In the detailed tasks, the numerical solutions of transitional phases, which are formulated based on literature (Czosnyka et al. 2011, Stadlbauer et al. 2010), must be validated on the quantitative assessment. The impact of the pulsatile flow parameters on CSF dynamics is investigated in detail such that the fundamental findings can be applied to physiological and engineering problems.
Where am I heading?
The outcomes have potential to be expanded in various areas within fluid mechanics and the numerical models of CNS. The quantitatively validated model can enable an optimized CSF therapeutics to realize the more rapid application for efficient clinical usage and the reduced cost for experiments. Together with the clinical researches of CSF flow, the high-resolution CFD analysis could lead to a better understanding of the pathophysiological basis of a number of diseases with dysfunction of CSF flow, e.g., normal pressure hydrocephalus and Alzheimer’s diseases.
Program 1 - CNS geometry impact on steady-streaming
The numerical simulation uses the brain ventricles and the cranial SAS geometry extracted from an MRI measurement to build up the basis of CSF modeling works. To support the project workflow, the flow visualization and the graphical data-manipulation tools are required. The CSF motion is analyzed at the steady-streaming condition which is closely related with the geometry of brain ventricular system connected to the cranial and the spinal SAS. At the beginning an isolated ventricular system is considered with the median aperture which drains the CSF from the fourth ventricle to SAS. Then the spinal SAS is also included to compute the impact of geometry variations on the flow field. The volume flow and the pressure distribution will be determined at the systolic and the diastolic phases. The hypothesized high-pressure distributions are configured to understand the CSF dynamics in abnormal conditions. The quantitative assessment includes the trade-off between accuracy and computational efficiency achieved by coarser meshes.
Program 2 - CSF dynamics at pulsatile conditions
Based on the flow parameters used in the steady-streaming condition, a pulsatile flow inside the ventricular system can be configured to mimic the physiological reality more closely. In this sub-project, the impact of pulsatile flows on the CSF dynamics is studied with varying pulsation frequencies. The spectral components are correlated with the flow instabilities which trigger the turbulence transition in the ventricles and SAS. The geometries of the spinal SAS as well as the ventricles are required to predict the CSF flow field in a healthy and a dysfunctional CNS. Due to the unsteady pulsatility and the large CSF spaces, the computational cost is significantly increased for a high-resolution numerical simulation. The quantitative assessment includes the trade-off between accuracy and computational efficiency achieved by coarser meshes.